- Calculating Moles With Avogadro's Number
- Calculating Moles With Avogadro's Numbers
- Avogadro's Number Calculator
- Why Is A Mole 6.022 X10 23
- Avogadro's Number Moles To Atoms
- Formula Avogadro's Number
- How To Calculate Mole Number
- The number of particles in 1 mole of any substance. 1mol of anything = 6.02x10 23. It can be used as a conversion factor from atoms to moles or moles to atoms. Avogadro's Number = 6.022x10 23 There are 6.022x10 23 atoms in 1 mole of atoms.
- The numeric value of the Avogadro constant expressed in reciprocal mole, a dimensionless number, is called the Avogadro number, sometimes denoted N or N 0, which is thus the number of particles that are contained in one mole, exactly 6.022 140 76 × 10 23.
More Chemistry Lessons
If you divide the charge on a mole of electrons by the charge on a single electron you obtain a value of Avogadro’s number of 6.02214154 x 10 23 particles per mole. Another approach to determining.
Stoichiometry Lessons
 In these lessons, we will learn the Molar Volume, Avogadro's Law, how to calculate gas volumes given moles and grams, how to calculate moles given gas volumes and how to calculate gas volumes given the chemical equation.
In these lessons, we will learn the Molar Volume, Avogadro's Law, how to calculate gas volumes given moles and grams, how to calculate moles given gas volumes and how to calculate gas volumes given the chemical equation. The molar volume is the volume occupied by one mole of a substance (chemical element or chemical compound) at a given temperature and pressure.
There are two standards, commonly used in schools:
- STP (standard temperature and pressure) which is 0° C and 1 atmosphere.
- RTP (room temperature and pressure) which is 25° C and 1 atmosphere.
Molar Volume
Avogadro’s Law states that:
Calculating Moles With Avogadro's Number
1 mole of every gas occupies the same volume, at the same temperature and pressure.
At STP (standard temperature and pressure), this volume is 22.4 liters
At RTP (room temperature and pressure), this volume is 24 dm3 (liters)
We can also say:
The molar volume of a gas is 22.4 liters at STP (standard temperature and pressure).
The molar volume of gas is 24 dm3 at RTP (room temperature and pressure).
The following diagrams show how to convert between Mass, Moles and Gas Volumes. Scroll down the page for more examples and solutions.How to find the molar volume of a gas using the ideal gas law?
The most common molar volume is the molar volume of an ideal gas at standard temperature and pressure (273 K and 1.00 atm).
The molar volume is the volume occupied by 1 mol of a gas at standard temperature and pressure (STP). It can be calculated using PV = nRT.
Gas volumes from moles and grams
Example:
Calculate the volume of carbon dioxide gas, CO2, occupied by (a) 5 moles and (b) 0.5 moles of the gas occupied at STP.
Solution:
a) Volume of CO2= number of moles of CO2 × 22.4 L
= 5 × 22.4
= 112 L
b) Volume of CO2
= number of moles of CO2 × 22.4 L
= 0.5 × 22.4
= 11.2 L
How to convert from grams to moles to liters?
The following video shows an example of grams to moles to liters conversion.
It shows how to convert grams of a substance to liters at STP.
Example:
What is the volume of 5.643g of COs at STP?
Moles from Gas Volume
Example:
Calculate the number of moles of ammonia gas, NH3, in a volume of 80 L of the gas measured at STP.
Solution:
Volume of gas = number of moles × 22.414 L/mol
How to convert from liters to moles?
The following video shows an example of liters to moles conversion. It shows how to convert litres of a gas at STP into moles
Example:
How many moles are there in 60.2L of COs at STP?
- Show Step-by-step Solutions
Gas volumes from equations
From the equation for a reaction, we can tell how many moles of a gas take part. Using Avogadro’s Law, we can also work out its volume.
Example:
What volume of hydrogen will react with 22.4 liters of oxygen to form water? (All volumes are measured at STP)
Solution:
Step 1: Write a balanced equation for the reaction.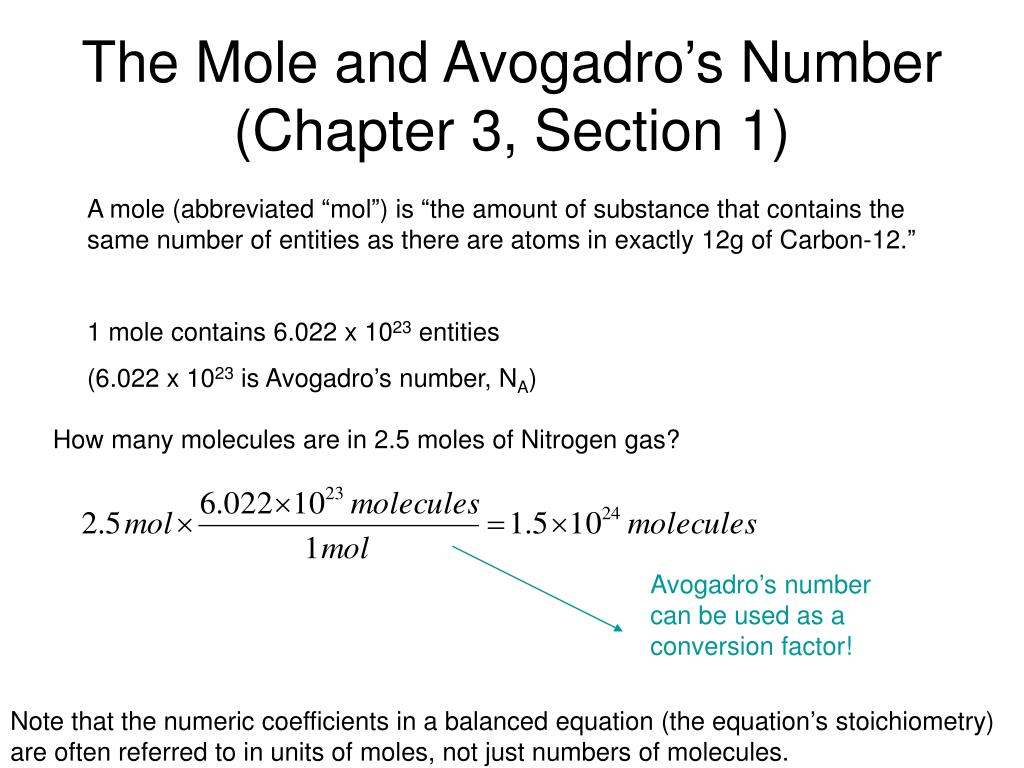 2H2 (g) + O2 (g) → 2H2O (l)
2H2 (g) + O2 (g) → 2H2O (l)Step 2: Calculate the volume.
From the equation, 2 volumes of hydrogen react with 1 of oxygen or
2 × 22.4 liters of hydrogen react with 22.4 liters of oxygen.
The volume of hydrogen that will react is 44.8 liters.
Example:
When sulfur burns in air it forms sulfur dioxide. What volume of this gas is produced when 1 g of sulfur burns? (Ar : S = 32) (All volumes are measured at STP)
Solution:
Step 1: Write a balanced equation for the reaction.S (s) + O2 (g) → SO2 (g)
Step 2: Get the number of moles from the grams.
32 g of sulfur atoms = 1 mole of sulfur atoms
Calculating Moles With Avogadro's Numbers
So, 1 g = 1 ÷ 32 mole or 0.03125 moles of sulfur atoms
1 mole of sulfur atoms gives 1 mole of sulfur dioxide molecules
 So, 0.03125 moles of sulfur atoms gives 0.03125 moles of sulfur dioxide
So, 0.03125 moles of sulfur atoms gives 0.03125 moles of sulfur dioxideAvogadro's Number Calculator
Step 3: Get the volume.
1 mole of sulfur dioxide molecules has a volume of 22.4 at STP
So, 0,03125 moles has a volume of 0.03125 × 22.4 = 0.7 liters at STP
 So, 0.7 liters of sulfur dioxide are produced.
So, 0.7 liters of sulfur dioxide are produced.How to solve equation stoichiometry questions with gases?
Examples and practice problems of solving equation stoichiometry questions with gases. We calculate moles with 22.4 L at STP, and use molar mass (molecular weight) and mole ratios to figure out how many products or reactants we have.
Example:
Why Is A Mole 6.022 X10 23
How many grams of H2
 O will be produced by 58.2L of CH4 at STP? Assume an excess of O2. Examples and practice problems of solving equation stoichiometry questions with gases. We calculate moles with the Ideal Gas Law, because the conditions are not at STP, and use molar mass (molecular weight) and mole ratios to figure out how many products or reactants we have.
O will be produced by 58.2L of CH4 at STP? Assume an excess of O2. Examples and practice problems of solving equation stoichiometry questions with gases. We calculate moles with the Ideal Gas Law, because the conditions are not at STP, and use molar mass (molecular weight) and mole ratios to figure out how many products or reactants we have.Example:
Avogadro's Number Moles To Atoms
If 85.0 g of NaN
Formula Avogadro's Number
3 decomposes at 75°C and 2.30 atm, what volume of N2How To Calculate Mole Number
will be made?- Show Step-by-step Solutions
Try the free Mathway calculator and problem solver below to practice various math topics. Try the given examples, or type in your own problem and check your answer with the step-by-step explanations.
We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.
