- Image showing periodicity of valence s-orbital radius for the chemical elements as size-coded balls on a periodic table grid. The R max values for neutral gaseous element valence orbitals are abstracted from reference 1.
- The small size of the fluorine atom makes it possible to pack a relatively large number of fluorine atoms or ions around a given coordination centre (central atom) where it forms many stable complexes—for example, hexafluorosilicate (SiF 6) 2− and hexafluoroaluminate (AlF 6) 3−. Fluorine is the most powerfully oxidizing element.
The fluoride ion, from the element fluorine, inhibits tooth decay. Fluorite (originally called fluorspar) crystals in daylight. The same fluorite crystals fluorescing in darkness after exposure to light. The phenomenon of fluorescence was given its name because it was first observed in fluorite. Having a chemical formula of F−, fluoride ion is the simplest inorganic, monatomic anion of fluorine with basic properties. It is considered a trace element. Fluoride ions are found in various minerals but are only present in trace amounts in water.
Click to see full answer.
Furthermore, does fluorine gain or lose electrons?
It can lose one of its electrons, making it an ion. It now has more positive protons than electrons so it has an overall positive charge. A fluorine atom will tend to gain, rather than lose, an electron. By gaining a negative electron, it has an overall negative charge.
Also, what happens when an atom gains an electron? However, if something happens to make an atom lose or gain an electron then the atom will no longer be neutral. An atom that gains or loses an electron becomes an ion. If it gains a negative electron, it becomes a negative ion. If it loses an electron it becomes a positive ion (see page 10 for more on ions).
how many electrons does fluorine gain or lose?
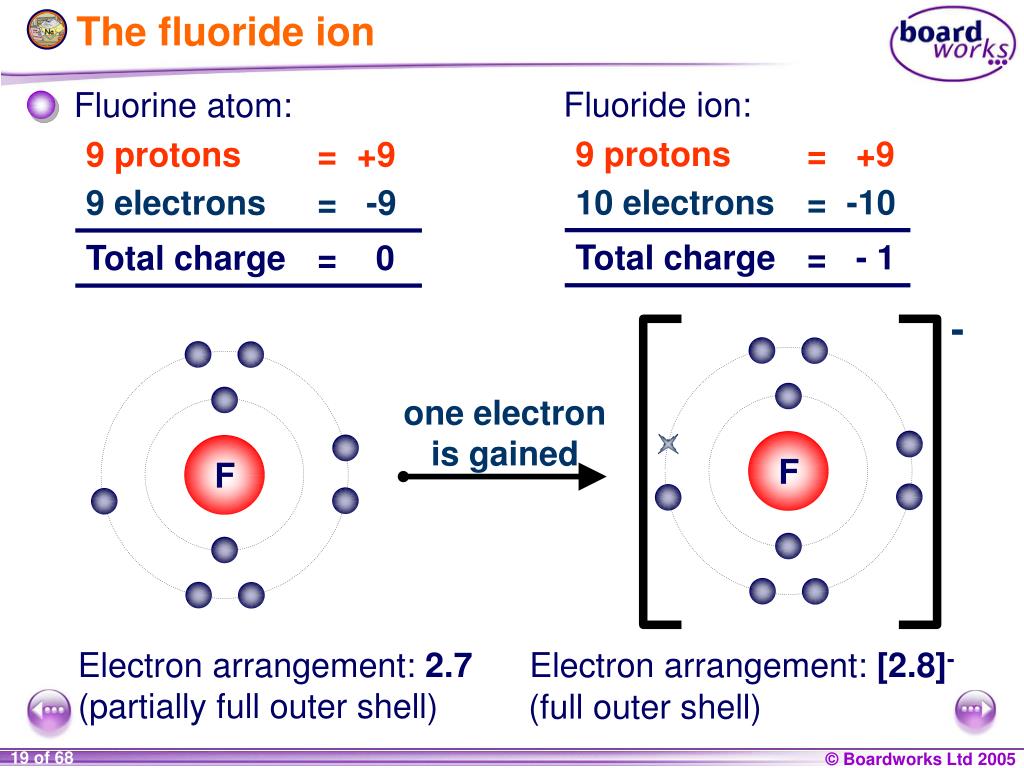
Example 1: A fluorine atom can get a full valence shell by either gaining one more electron, or by losing seven electrons. The former requires the transfer of less electrons, so the fluorine atom will try to gain one electron first. Therefore, F− ions are more common than F7+ ions.
What happens when fluorine atoms react?
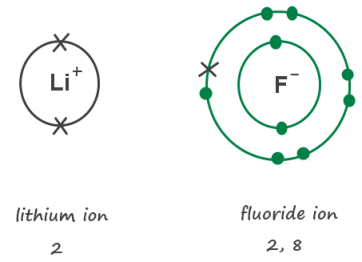
Fluorine is in Group 7. It has seven electrons in its outer shell. It gains an electron from another atom in reactions, forming a fluoride ion, F-. A fluoride ion has the same electronic structure as a neon atom (Ne).
- Formula: F2
- Molecular weight: 37.9968064
- IUPAC Standard InChI:
- InChI=1S/F2/c1-2
- Download the identifier in a file.
- IUPAC Standard InChIKey:PXGOKWXKJXAPGV-UHFFFAOYSA-N
- CAS Registry Number: 7782-41-4
- Chemical structure:
This structure is also available as a 2d Mol fileor as a computed3d SD file
The 3d structure may be viewed usingJavaorJavascript. - Permanent link for this species. Use this link for bookmarking this speciesfor future reference.
- Information on this page:
- Other data available:
- Data at other public NIST sites:
- Options:
Data at NIST subscription sites:
NIST subscription sites provide data under theNIST Standard ReferenceData Program, but require an annual fee to access.The purpose of the fee is to recover costs associatedwith the development of data collections included insuch sites. Your institution may already be a subscriber.Follow the links above to find out more about the datain these sites and their terms of usage.
Gas phase ion energetics data
Go To:Top, References, Notes
Data compilation copyrightby the U.S. Secretary of Commerce on behalf of the U.S.A.All rights reserved.
Data evaluated as indicated in comments:
HL - Edward P. Hunter and Sharon G. Lias
L - Sharon G. Lias
Data compiled as indicated in comments:
LBLHLM - Sharon G. Lias, John E. Bartmess, Joel F. Liebman, John L. Holmes, Rhoda D. Levin, and W. Gary Mallard
LLK - Sharon G. Lias, Rhoda D. Levin, and Sherif A. Kafafi
RDSH - Henry M. Rosenstock, Keith Draxl, Bruce W. Steiner, and John T. Herron
LL - Sharon G. Lias and Joel F. Liebman
B - John E. Bartmess
| Quantity | Value | Units | Method | Reference | Comment |
|---|---|---|---|---|---|
| IE (evaluated) | 15.697 ± 0.003 | eV | N/A | N/A | L |
| Quantity | Value | Units | Method | Reference | Comment |
| Proton affinity (review) | 332. | kJ/mol | N/A | Hunter and Lias, 1998 | HL |
| Quantity | Value | Units | Method | Reference | Comment |
| Gas basicity | 305.5 | kJ/mol | N/A | Hunter and Lias, 1998 | HL |
Electron affinity determinations
| EA (eV) | Method | Reference | Comment |
|---|---|---|---|
| 3.005 ± 0.071 | R-A | Wenthold and Squires, 1995 | EA fixed at 0K value, not 298K of heat of formation; B |
| 3.120 ± 0.070 | CIDC | Artau, Nizzi, et al., 2000 | B |
| 3.07998 | ECD | Ayala, Wentworth, et al., 1981 | Vertical Detachment Energy: 1.24 eV; B |
| 2.94 ± 0.20 | EIAE | Harland and Franklin, 1974 | From NF3; B |
| 2.90 ± 0.22 | EIAE | DeCorpo and Franklin, 1971 | From BF3; B |
| 3.16558 | EIAE | Wang and Franklin, 1980 | From SO2F2; B |
| >2.80 ± 0.30 | EIAE | Thynne, 1972 | From CF2O; B |
| 3.08 ± 0.10 | Endo | Chupka, Berkowitz, et al., 1971 | B |
| >2.99997 | EIAE | Reese, Dibeter, et al., 1958 | From SO2F2; B |
Ionization energy determinations
| IE (eV) | Method | Reference | Comment |
|---|---|---|---|
| 15.697 ± 0.003 | PE | Van Lonkhuyzen and De Lange, 1984 | LBLHLM |
| 15.70 | PE | Bieri, Schmelzer, et al., 1980 | LLK |
| 15.694 | TE | Guyon, Spohr, et al., 1976 | LLK |
| 15.70 ± 0.02 | S | Gole and Margrave, 1972 | LLK |
| 15.70 ± 0.01 | PE | Potts and Price, 1971 | LLK |
| 15.70 | PE | Cornford, Frost, et al., 1971 | LLK |
| 15.74 | PE | Cornford, Frost, et al., 1971 | LLK |
| 15.686 ± 0.006 | PI | Berkowitz, Chupka, et al., 1971 | LLK |
| 15.70 | PE | Anderson, Mamantov, et al., 1971 | LLK |
| 15.69 ± 0.01 | PI | Dibeler, Walker, et al., 1969 | RDSH |
| 15.7 | S | Iczkowski and Margrave, 1959 | RDSH |
| 15.70 | PE | Dyke, Josland, et al., 1984 | Vertical value; LBLHLM |
Appearance energy determinations
| Ion | AE (eV) | Other Products | Method | Reference | Comment |
|---|---|---|---|---|---|
| F+ | 15.2 | F- | EI | Veljkovic, Neskovic, et al., 1992 | LL |
| F+ | 19.008 | F | PI | Berkowitz and Wahl, 1973 | LLK |
| F+ | 15.6 | F- | PI | Berkowitz, Chupka, et al., 1971 | LLK |
| F+ | 19.008 | F | PI | Berkowitz, Chupka, et al., 1971, 2 | LLK |
| F+ | 15.48 | F- | PI | Dibeler, Walker, et al., 1969 | RDSH |

References
Go To:Top, Gas phase ion energetics data, Notes
Data compilation copyrightby the U.S. Secretary of Commerce on behalf of the U.S.A.All rights reserved.
Hunter and Lias, 1998
Hunter, E.P.; Lias, S.G.,Evaluated Gas Phase Basicities and Proton Affinities of Molecules: An Update,J. Phys. Chem. Ref. Data, 1998, 27, 3, 413-656, https://doi.org/10.1063/1.556018. [all data]
Fluorine Ion That Will Form
Wenthold and Squires, 1995
Wenthold, P.G.; Squires, R.R.,Bond dissociation energies of F2(-) and HF2(-). A gas-phase experimental and G2 theoretical study,J. Phys. Chem., 1995, 99, 7, 2002, https://doi.org/10.1021/j100007a034. [all data]
Artau, Nizzi, et al., 2000
Artau, A.; Nizzi, K.E.; Hill, B.T.; Sunderlin, L.S.; Wenthold, P.G.,Bond dissociation energy in trifluoride ion,J. Am. Chem. Soc., 2000, 122, 43, 10667-10670, https://doi.org/10.1021/ja001613e. [all data]
Ayala, Wentworth, et al., 1981
Ayala, J.A.; Wentworth, W.E.; Chen, E.C.M.,Electron attachment to halogens,J. Phys. Chem., 1981, 85, 768. [all data]
Harland and Franklin, 1974
Harland, P.W.; Franklin, J.L.,Partitioning of excess energy in dissociative resonance capture processes,J. Chem. Phys., 1974, 61, 1621. [all data]
DeCorpo and Franklin, 1971
DeCorpo, J.J.; Franklin, J.L.,Electron affinities of the halogen molecules by dissociative electron attachment,J. Chem. Phys., 1971, 54, 1885. [all data]
Wang and Franklin, 1980
Wang, J.-S.; Franklin, J.L.,Reactions and energy distributions in dissociative electron capture processes in sulfuryl halides,Int. J. Mass Spectrom. Ion Phys., 1980, 36, 233. [all data]
Thynne, 1972
Thynne, J.C.J.,Negative Ion Studies with a Time-of-Flight Mass Spectrometer.,Dyn. Mass Spectrom., 1972, 3, 67. [all data]
Chupka, Berkowitz, et al., 1971
Chupka, W.A.; Berkowitz, J.; Gutman, D.,Electron Affinities of Halogen Diatomic Molecules as Determined by Endoergic Charge Exchange,J. Chem. Phys., 1971, 55, 6, 2724, https://doi.org/10.1063/1.1676487. [all data]
Reese, Dibeter, et al., 1958
Reese, R.M.; Dibeter, V.H.; Franklin, J.L.,Electron impact studies of sulfur dioxide and sulfuryl fluoride,J. Chem. Phys., 1958, 29, 880. [all data]
Van Lonkhuyzen and De Lange, 1984
Van Lonkhuyzen, H.; De Lange, C.A.,High-resolution UV photoelectron spectroscopy of diatomic halogens,Chem. Phys., 1984, 89, 313. [all data]
Bieri, Schmelzer, et al., 1980
Bieri, G.; Schmelzer, A.; Asbrink, L.; Jonsson, M.,Fluorine and the fluoroderivatives of acetylene and diacetylene studied by 30.4 nm He(II) photoelectron spectroscopy,Chem. Phys., 1980, 49, 213. [all data]
Guyon, Spohr, et al., 1976
Guyon, P.-M.; Spohr, R.; Chupka, W.A.; Berkowitz, J.,Threshold photoelectron spectra of HF, DF, F2,J. Chem. Phys., 1976, 65, 1650. [all data]
Fluorine Ion Electrons
Gole and Margrave, 1972
Gole, J.L.; Margrave, J.L.,The vacuum ultraviolet spectrum of molecular fluorine,J. Mol. Spectrosc., 1972, 43, 65. [all data]
Fluoride Ion Batteries
Potts and Price, 1971
Potts, A.W.; Price, W.C.,Photoelectron spectra of the halogens and mixed halides ICI and lBr,J. Chem. Soc. Faraday Trans., 1971, 67, 1242. [all data]
Cornford, Frost, et al., 1971
Cornford, A.B.; Frost, D.C.; McDowell, C.A.; Ragle, J.L.; Stenhouse, I.A.,Photoelectron spectra of the halogens,J. Chem. Phys., 1971, 54, 2651. [all data]
Berkowitz, Chupka, et al., 1971
Berkowitz, J.; Chupka, W.A.; Guyon, P.M.; Holloway, J.H.; Spohr, R.,Photoionization mass spectrometric study of F2, HF, and DF,J. Chem. Phys., 1971, 54, 5165. [all data]
Anderson, Mamantov, et al., 1971
Anderson, C.P.; Mamantov, G.; Bull, W.E.; Grimm, F.A.; Carver, J.C.; Carlson, T.A.,Photoelectron spectrum of chlorine monofluoride,Chem. Phys. Lett., 1971, 12, 137. [all data]
Dibeler, Walker, et al., 1969
Dibeler, V.H.; Walker, J.A.; McCulloh, K.E.,Dissociation energy of fluorine,J. Chem. Phys., 1969, 50, 4592. [all data]
Iczkowski and Margrave, 1959
Iczkowski, R.P.; Margrave, J.L.,Absorption spectrum of fluorine in the vacuum ultraviolet,J. Chem. Phys., 1959, 30, 403. [all data]
Dyke, Josland, et al., 1984
Dyke, J.M.; Josland, G.D.; Snijders, J.G.; Boerrigter, P.M.,Ionization energies of the diatomic halogens and interhalogens studied with relativistic hartree-fock-slater calculations,Chem. Phys., 1984, 91, 419. [all data]
Fluorine Ion Protons Neutrons Electrons
Veljkovic, Neskovic, et al., 1992
Veljkovic, M.V.; Neskovic, O.M.; Zmbov, K.F.,Mass spectrometric study of the thermal decomposition of F2,J. Serb. Chem. Soc., 1992, 57, 753. [all data]
Berkowitz and Wahl, 1973
Berkowitz, J.; Wahl, A.C.,The dissociation energy of fluorine,Adv. Fluorine Chem., 1973, 7, 147. [all data]
Berkowitz, Chupka, et al., 1971, 2
Berkowitz, J.; Chupka, W.A.; Guyon, P.M.; Holloway, J.; Spohr, R.,Photo-ionization studies of F2, HF, DF, and the xenon fluorides,Advan. Mass Spectrom., 1971, 5, 112. [all data]
Notes
Go To:Top, Gas phase ion energetics data, References
- Symbols used in this document:
AE Appearance energy EA Electron affinity IE (evaluated) Recommended ionization energy - Data from NIST Standard Reference Database 69:NIST Chemistry WebBook
- The National Institute of Standards and Technology (NIST)uses its best efforts to deliver a high quality copy of theDatabase and to verify that the data contained therein havebeen selected on the basis of sound scientific judgment.However, NIST makes no warranties to that effect, and NISTshall not be liable for any damage that may result fromerrors or omissions in the Database.
- Customer supportfor NIST Standard Reference Data products.
